ROADSAVER™ Carotid Stent System
LEADING THE EVOLUTION OF CAROTID ARTERY STENTING
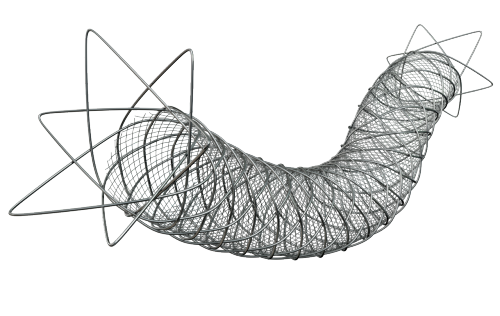
The next-generation design of the ROADSAVER Stent System features an innovative micro-mesh technology which empowers clinicians to safely and effectively treat carotid artery disease (CAD) in an expanding patient population.
OPERATOR EFFICIENCY
Demonstrated shorter procedural times when compared to WALLSTENT, Precise Pro, CGUARD and XACT.*1
INNOVATIVE DESIGN
Dual layer micro-mesh designed to restore flow and contain plaque1,2
Maintains high plaque scaffolding while maintaining conformability and stability, even in complex anatomy1,2
REDUCE COMPLICATIONS
30-day low
complication rates294.9% procedural
success rate2
ADVANCING CAROTID STENTING
The ROADSAVER Stent System takes performance to the next level.
The ROADSAVER Carotid Stent System is indicated for use with the NANOPARASOLTM Embolic Protection System.
NANOPARASOL EMBOLIC PROTECTION SYSTEM
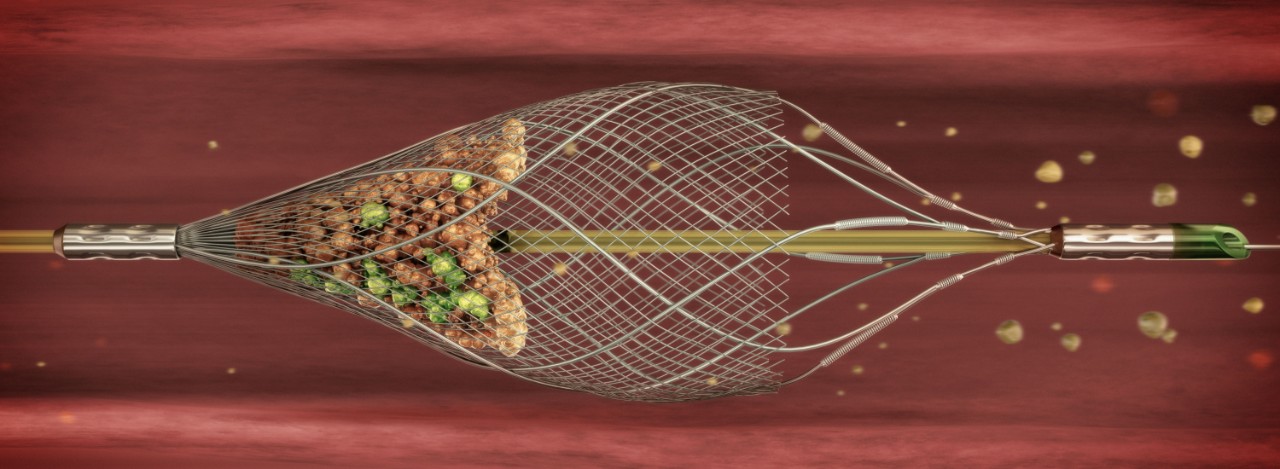
The NANOPARASOL system is an embolic protection device designed to capture and remove dislodged debris during a carotid artery interventional procedure.1
CLINICAL INFORMATION
The ROADSAVER Carotid Stent System offers technical improvements relative to single-layer carotid stents, including inner micromesh layer ensuring sustained embolic protection during and after CAS and flexible low-crossing profile delivery system, good in-vessel flexibility and good wall apposition in tortuous anatomies.1-4.
Clinical performance of the ROADSAVER Stent System demonstrated a high procedure success rate, operator specialty independence and is supported by evidence confirming that these technical advancements translate into favorable safety and efficacy profile of the device.1-4
The ROADSAVER Stent System is supported by three key registration studies, one of which is the largest real-world study performed on a single-layer dual layer micro mesh stent.2,5,6
Carotid Stent Trial to Evaluate the Safety and Efficacy of the ROADSAVER™ Stent Used in Conjunction with the NANOPARASOL™ Embolic Protection System for Patients at Increased Risk for Adverse Events2
A prospective, multicenter, single-arm, open label clinical study:
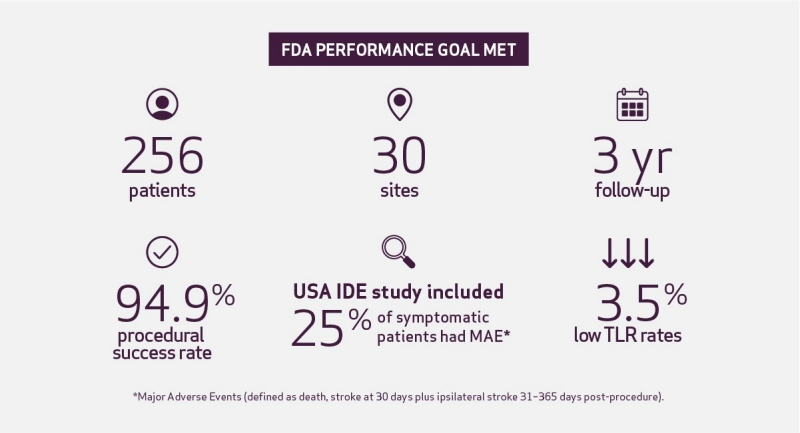
Conclusion: The event rate for ipsilateral stroke up to 1-year post-procedure was 2.7%, the primary endpoint rate (MAE at 30 days plus ipsilateral stroke 1-year post-procedure) was 5.9%.
Clinical trial of carotid artery stenting using dual-layer CASPER (ROADSAVER) stent for carotid endarterectomy in patients at high and normal risk in the Japanese population.5

Conclusion: The MAE rate following use of the CASPER (ROADSAVER) stent was low (1.4%). The MAE rate in patients with normal CEA risk was 1.2%5
Real-World Study of a Dual-Layer Micromesh Stent in Elective Treatment of Symptomatic and Asymptomatic Carotid Artery Stenosis (ROADSAVER)6
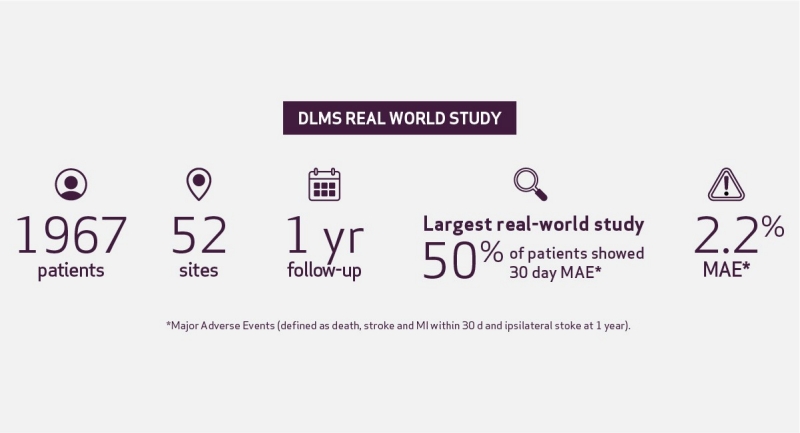
Conclusion: Low 30-day MAE rate 2.2%, low 1-year stroke related any death and ipsilateral stroke, Low TLR rate .8%.6
ADDITIONAL ROADSAVER CLINICAL INFORMATION
Incidence of New Ischaemic Brain Lesions After Carotid Artery Stenting with the Micromesh ROADSAVER Carotid Artery Stent: A Prospective Single-Centre Study7
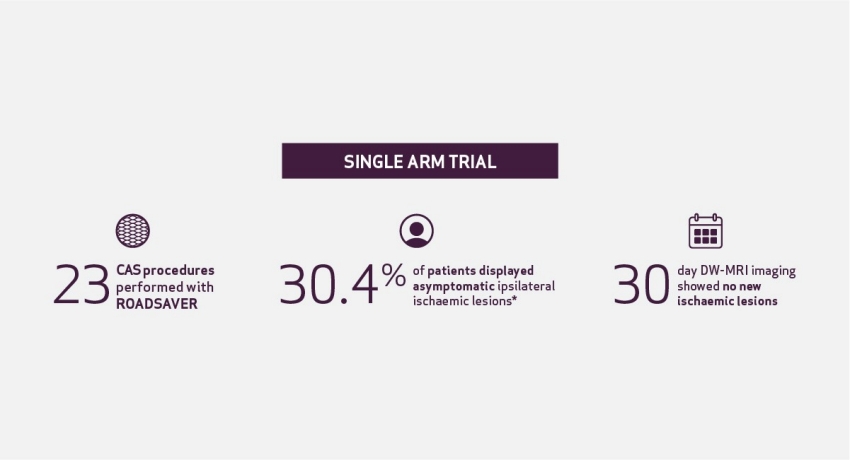
Conclusion: The study showed protected effect of micromesh ROADSAVER stent during and post CAS procedure.7
Carotid Wallstent Versus ROADSAVER Stent and Distal Versus Proximal Protection on Cerebral Microembolization During Carotid Artery Stenting8
- Randomly compared the double-layer Roadsaver stent (RS) with the single-layer Carotid Wallstent (CW), 104 consecutive patients
- Patients presented with symptomatic/asymptomatic carotid artery stenosis with lipid rick plaque ( as diagnosed by CTA) were double randomized: based on the types of embolic protection device (Mo.Ma proximal protection device vs. FilterWire distal protection device) and type of stent: Wallstent (representing 1st generation CAS) vs Roadsaver ( representing 2nd generation CAS).
- All phases of the procedure were followed with Transcranial Doppler. (TCD) and micro embolic signals count was analyzed.
- In patients with high-risk, lipid-rich plaque undergoing CAS combination of Mo.Ma proximal protection device and Roadsaver CAS led to the lowest microembolic signals count on TCD.
Conclusion: No significant differences in baseline characteristics were found among the four groups.8
Prospective evaluation of acute cerebral injury by DW-MRI following transcarotid artery revascularization using a double-layer micromesh stent9
- A prospective, single-center, single-arm trial including 85 patients ( 75% symptomatic with mean age of 72 yr) that underwent TCAR with the Roadsaver stent
- The 2-3 days post-procedure DW MRI was performed in 83/85 pts showed 10.8% of new lesions. All lesions were asymptomatic.
- 30 days results showed no stoke or death, and 2 patients had MI.
Conclusion: The study showed that the use of TCAR and ROADSAVER could be a safe alternative to CAE even in symptomatic and elderly patients.9
REIMBURSEMENT
More Patients. More Options.
As expanded coverage of carotid artery stents shifts, the ROADSAVER Stent System offers the configuration options for treating more patients.
Expanded CMS guidelines2 now include coverage of:
• Patients with symptomatic carotid artery stenosis ≥50%
• Patients with asymptomatic carotid artery stenosis ≥70%
Email: Reimbursement@terumomedical.com
Contact: 855-380-3081
PRODUCT CODES
ROADSAVER Cartoid Artery Stent System
| Product Code | Product Description | Unconstrained Dimensions (mm) | Implanted Dimensions (mm) | |||||||
| Vessel Ø 1 mm Smaller than Unconstrained Ø | Vessel Ø 2 mm Smaller than Unconstrained Ø | |||||||||
| Diameter | Overall Length | Dual Layer Length | Vessel Diameter | Dual Layer Length | Overall Length | Vessel Diameter | Dual Layer Length | Overall Length | ||
| RDS-0520-143RX | 5mm x 20mm x 143cm | 5 | 25 | 20 | 4 | 20 | 33 | 3.5* | 25 | 34 |
| RDS-0530-143RX | 5mm x 30mm x 143cm | 5 | 37 | 30 | 4 | 35 | 47 | 3.5* | 40 | 50 |
| RDS-0540-143RX | 5mm x 40mm x 143cm | 5 | 47 | 40 | 4 | 45 | 59 | 3.5* | 52 | 62 |
| RDS-0616-143RX | 6mm x 16mm x 143cm | 6 | 22 | 16 | 5 | 20 | 32 | 4 | 23 | 35 |
| RDS-0625-143RX | 6mm x 25mm x 143cm | 6 | 33 | 25 | 5 | 30 | 44 | 4 | 33 | 48 |
| RDS-0630-143RX | 6mm x 30mm x 143cm | 6 | 40 | 30 | 5 | 40 | 53 | 4 | 43 | 58 |
| RDS-0718-143RX | 7mm x 18mm x 143cm | 7 | 25 | 18 | 6 | 23 | 35 | 5 | 26 | 38 |
| RDS-0725-143RX | 7mm x 25mm x 143cm | 7 | 35 | 25 | 6 | 30 | 47 | 5 | 36 | 52 |
| RDS-0730-143RX | 7mm x 30mm x 143cm | 7 | 40 | 30 | 6 | 40 | 53 | 5 | 44 | 60 |
| RDS-0820-143RX | 8mm x 20mm x 143cm | 8 | 25 | 20 | 7 | 25 | 36 | 6 | 27 | 40 |
| RDS-0825-143RX | 8mm x 25mm x 143cm | 8 | 35 | 25 | 7 | 30 | 49 | 6 | 38 | 54 |
| RDS-0830-143RX | 8mm x 30mm x 143cm | 8 | 40 | 30 | 7 | 40 | 55 | 6 | 45 | 61 |
| RDS-0840-143RX | 8mm x 40mm x 143cm | 8 | 47 | 40 | 7 | 50 | 67 | 6 | 60 | 75 |
| RDS-0920-143RX | 9mm x 20mm x 143cm | 9 | 33 | 20 | 8 | 30 | 45 | 7 | 33 | 48 |
| RDS-0930-143RX | 9mm x 30mm x 143cm | 9 | 40 | 30 | 8 | 40 | 55 | 7 | 45 | 60 |
| RDS-1020-143RX | 10mm x 20mm x 143cm | 10 | 35 | 20 | 9 | 30 | 45 | 8 | 35 | 50 |
| RDS-1030-143RX | 10mm x 30mm x 143cm | 10 | 43 | 30 | 9 | 40 | 55 | 8 | 45 | 60 |
*5 mm stents maximumum oversizing is limited to 1.5mm
NANOPARASOL™ Embolic Protection System
| MODEL NUMBER | LABELED FILTER SIZE | UNCONSTRAINED FILTER OD (mm) | REFERENCE VESSEL DIAMETERS (mm) | |||||||
| PNP4514C-190 | SMALL | 5.2 | 3.0 – 4.5 | |||||||
| PNP6514C-190 | LARGE | 7.2 | 4.5 – 6.5 | |||||||
DOCUMENTS
FREQUENTLY ASKED QUESTIONS
Q: Carotid stent MRI safety: Is the ROADSAVER carotid stent system MRI-safe?
A: The ROADSAVER Carotid Stent System is MRI Conditional. This means that a patient implanted with the device may undergo MRI scans only under specific conditions. Scanning outside of these conditions may result in serious patient injury. Patients are instructed to refer to their patient implant card for additional information.
For complete MRI safety information, please consult the MRI Safety information section of the ROADSAVER Stent System Instructions for Use (IFU).
ASSOCIATED PRODUCTS
ROADSAVER Carotid Stent System INDICATIONS FOR USE
The ROADSAVER Carotid Stent System, when used in conjunction with the Nanoparasol Embolic Protection System (EPS), is indicated for the treatment of carotid artery stenosis in patients with elevated risk for adverse events following carotid endarterectomy and meet the criteria outlined below:
1. Patients who have either de novo atherosclerotic or post endarterectomy restenotic lesion(s) in the internal carotid arteries or at the carotid bifurcation with ≥50% stenosis if symptomatic or ≥80% stenosis if asymptomatic (both defined by angiography),
AND
2. Patients having a vessel with reference diameters between 3.5 mm and 9.0 mm at the target lesion.
CONTRAINDICATIONS
The ROADSAVER Carotid Stent System is contraindicated for use in:
· Patients in whom anticoagulant, antiplatelet therapy or thrombolytic drugs are contraindicated
· Patients with known hypersensitivity to nickel-titanium
· Patients with severe vascular tortuosity or anatomy that would preclude the safe introduction of a guide catheter, sheath, embolic protection system, or stent system
· Patients with uncorrected bleeding disorders
· Lesions in the ostium of the common carotid artery
· Carotid vessel with <25mm gap between the target location of the distal end of the stent and the proximal end of the distal protection device.
NANOPARASOL Embolic Protection System INDICATIONS FOR USE:
The NANOPARASOL EPS is indicated for use as a guidewire to contain and remove embolic material (thrombus/debris) while performing angioplasty and stenting procedures in carotid arteries. The diameter of the artery at the site of the filter placement should be between 3.0 and 6.5 mm.
CONTRAINDICATIONS
The NANOPARASOL Embolic Protection System is contraindicated for use in:
· Patients in whom anticoagulant, antiplatelet therapy or thrombolytic drugs is contraindicated
· Patients with known hypersensitivity to nickel-titanium
· Patients with severe vascular tortuosity or anatomy that would preclude the landing zone requirement or the safe introduction of a guide wire, guide catheter, introducer sheath, an embolic protection device, delivery catheter, or retrieval catheter
· Patients with uncorrected bleeding disorders
· Lesions in the ostium of the common carotid artery
REFERENCES
1. Data on File.
2. Siddiqui, Metzger, Schneider. Evaluation of the Roadsaver stent used in conjunction with the Nanoparasol embolic protection system for carotid artery stenosis. Carotid Endarterectomy. 2021 July 22; 1-123. Siddiqui A, et al. ClinicalTrials.gov identifier: NCT02657707.
3. https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=311. Accessed 3/26/25.
4. Kahlberg, Bilmanm, et al., Contemporary Results of Carotid Artery Stenting Using Low-Profile Dual-Metal Layer Nitinol Micromesh Stents in Relation to Single-Layer Carotid Stents. Journal of Endovascular Therapy 1–11, 2021.
5. Imamura, Nobuyuki, Yasushi, et. al. Clinical trial of carotid artery stenting using dual-layer CASPER (Roadsaver) stent for carotid endarterectomy in patients at high and normal risk in the Japanese population. Journal of NeuroInterventional Surgery vol 13, no. 6, 2021.
6. Sasko, Muller-Hulsbeck, Langhoff. Real-World Study of a Dual-Layer Micromesh Stent in Elective Treatment of Symptomatic and Asymptomatic Carotid Artery Stenosis (ROADSAVER). Cardiovascular Interventional Radiology 2022Mar; 45(3):277-282.
7. Ruffino, Faletti, Bergamasco et. al. Incidence of New Ischaemic Brain Lesions After Carotid Artery Stenting with the Micromesh Roadsaver Carotid Artery Stent: A Prospective Single-Centre Study. Cardiovascular and Interventional Radiology. 2016 Nov; 39(11):1541-1549.
8. Montorsi, Caputi, Galli, et. al. Carotid Wallstent Versus Roadsaver Stent and Distal Versus Proximal Protection on Cerebral Microembolization During Carotid Artery Stenting JACC Cardiovascular Interventions 2020 Feb 24; 12(4):403-414.
9. Lamarca, Flores, Martin, et. al. Prospective evaluation of acute cerebral injury by DW-MRI following transcarotid artery revascularization using a double-layer micromesh stent. Journal of Cardiovascular Surgery; 2023 December; 64(6):583-90.
*Data derived from the referenced OUS study and may not be representative of Confidence clinical study performance.
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
©2025 Terumo Medical Corporation. All rights reserved. All brand names are trademarks or registered trademarks of Terumo.