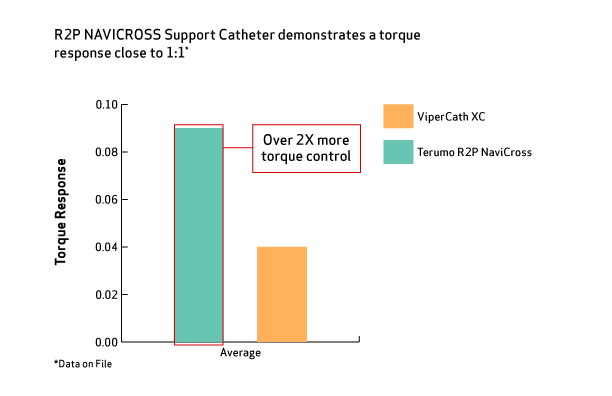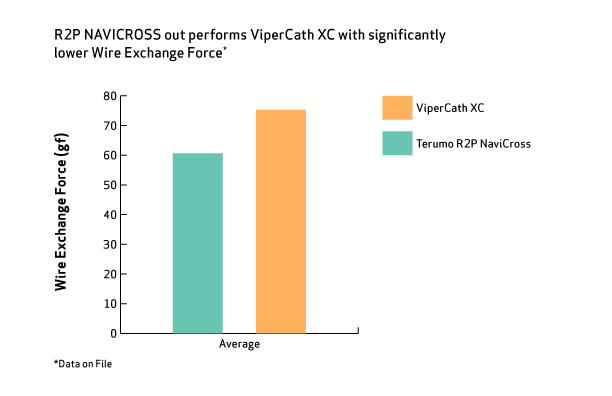NAVICROSS® Support Catheters
Your First-Choice Peripheral Support Catheter with Best-in-Class Performance1
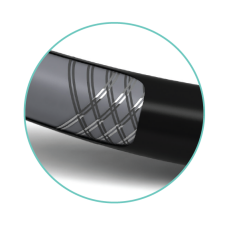
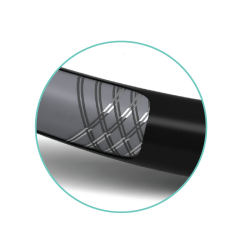
RADIOPAQUE MARKERS
Facilitate accurate assessment of lesion and stent positioning
Initial marker is 1 mm from distal tip: 40 mm and 60 mm spacing
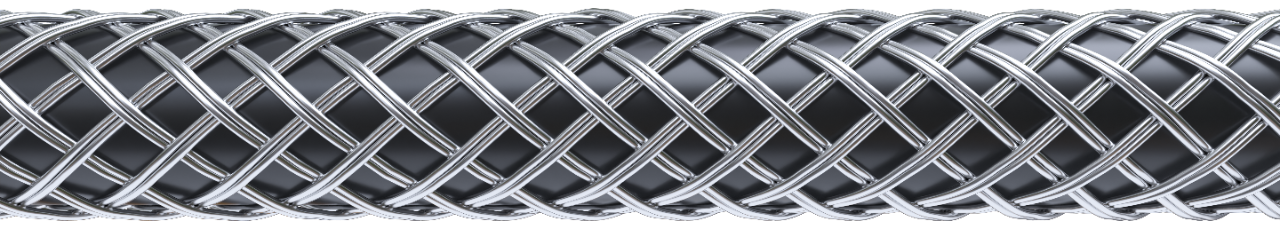
STAINLESS STEEL CONSTRUCTION
Double braided stainless steel engineered for optimal pushability1
ACCESS SITE
Available in three formats for all your femoral & radial access needs
NAVICROSS 0.018 – 65 cm, 90 cm, 135 cm, 150 cm
NAVICROSS 0.035 – 65 cm, 90 cm, 135 cm, 150 cm
R2P NAVICROSS 0.035 – 200 cm
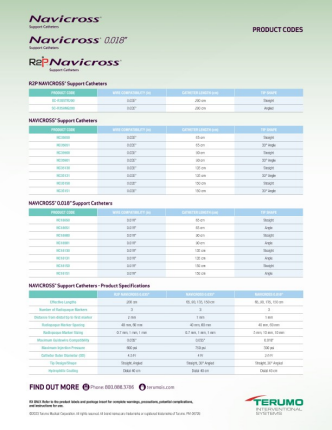
PRODUCT SPECIFICATIONS
| R2P NAVICROSS | NAVICROSS 0.018" | NAVICROSS 0.035" | |
| Effective Lengths | 200 cm | 65 cm, 90 cm, 135 cm, 150 cm | 65 cm, 90 cm, 135 cm, 150 cm |
| Number of Radiopaque Markers | 3 | 3 | 3 |
| Distance From Distal Tip To First Radiopaque Marker | 2 mm | 1 mm | 1 mm |
| Radiopaque Marker Spacing | 40 mm, 60 mm | 40 mm, 60 mm | 40 mm, 60 mm |
| Radiopaque Marker Sizing (Distal/Middle/Proximal) | 0.7 mm, 1 mm | 5 mm, 10 mm, 10 mm | 0.7 mm, 1 mm, 1 mm |
| Guidewire Compatibility | 0.035" | 0.018" | 0.035" |
| Maximum Injection Pressure | 600 psi | 300 psi | 750 psi |
| Catheter Outer Diameter (OD) | 1.48 mm | 0.85 mm | 1.39 mm |
| Minimum Introducer Sheath Compatibility | 4.5 Fr. | 2.6 Fr | 4 Fr |
| Tip Design/Shape | Straight, Angled | Straight, Angled | Straight, Angled |
| Hydrophilic Coating | Distal 40 cm | Distal 40 cm | Distal 40 cm |
PRODUCT CODES
NAVICROSS® Product Codes
| Tip Shape | Product Code | Wire Compatibility | Catheter Length |
| Straight | NC18650 | 0.018" | 65 cm |
| NC18900 | 90 cm | ||
| NC18130 | 135 cm | ||
| NC18150 | 150 cm | ||
| NC35650 | 0.035" | 65 cm | |
| NC35900 | 90 cm | ||
| NC35130 | 135 cm | ||
| NC35150 | 150 cm | ||
| SC-R35STR200 | 200 cm | ||
| Angle | NC18651 | 0.018" | 65 cm |
| NC18901 | 90 cm | ||
| NC18131 | 135 cm | ||
| NC18151 | 150 cm | ||
| NC35651 | 0.035" | 65 cm | |
| NC35901 | 90 cm | ||
| NC35131 | 135 cm | ||
| NC35151 | 150 cm | ||
| SC-R35ANG200 | 200 cm |