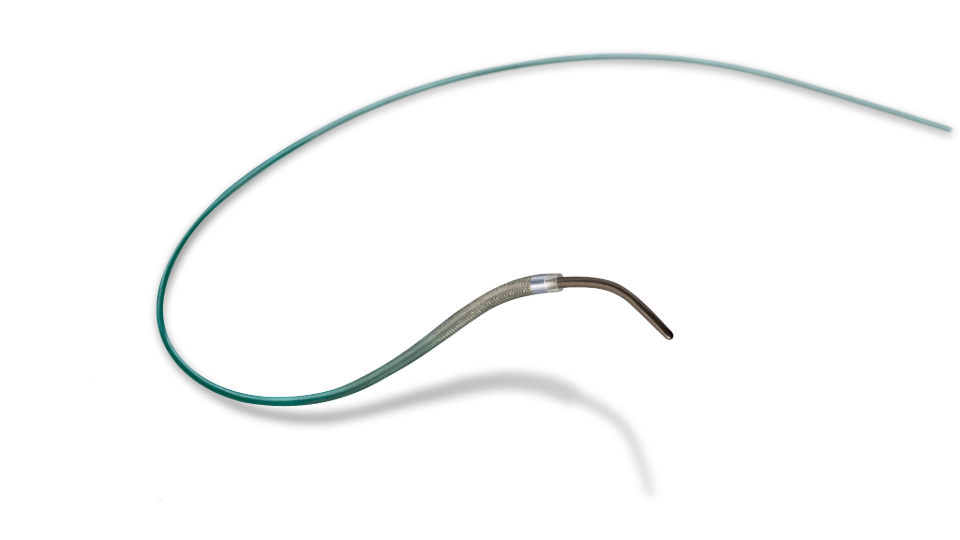
PROGREAT® Microcatheters
PROGREAT® Microcatheters are designed to allow navigation through tortuous vasculature to access peripheral vessels, with optimal trackability and delivery of therapeutic agents for embolization.
USE WITH CONFIDENCE
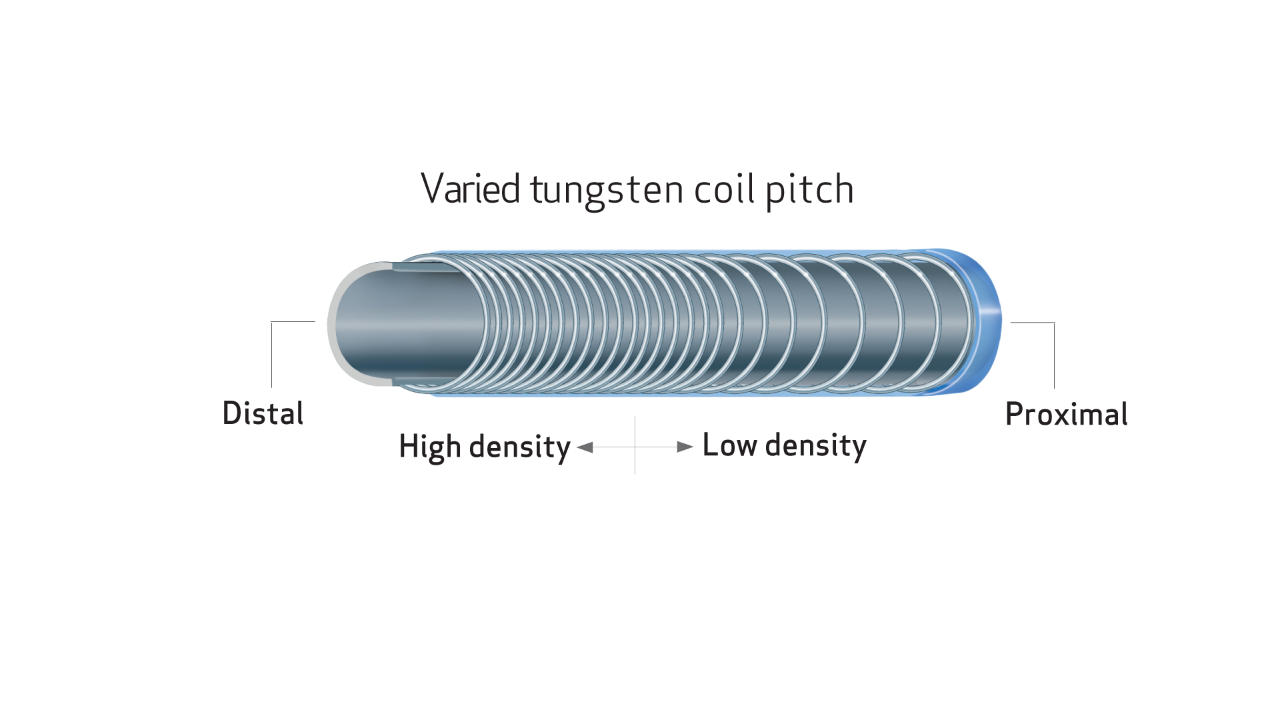
Unique tungsten coil layer helps maintain device lumen integrity.
INCREASE EFFICIENCY
Microcatheter and guidewire together reduces procedural steps to save time1.
REDUCE COMPLICATIONS
PTFE inner layer allows for smooth passage of embolic agents including coils, microspheres and PVA particles2.
PROGREAT: ADVANCED MICROCATHETER SOLUTIONS FOR PRECISION VASCULAR ACCESS
DESIGNED TO CONFIDENTLY DELIVER EMBOLOTHERAPY
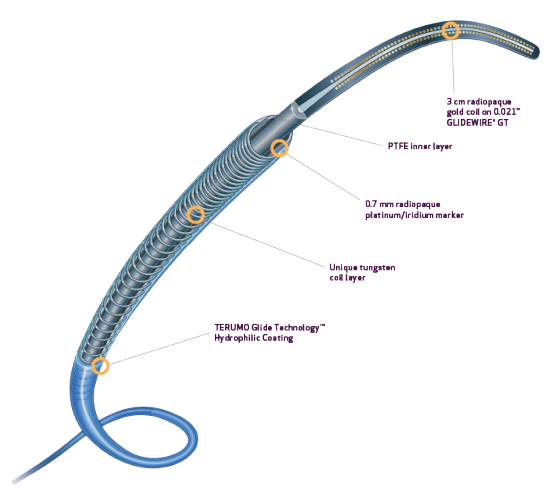
- Tungsten coil reinforcement for kink resistance2
- PTFE inner layer is designed for exceptional guidewire tracking, with virtually frictionless delivery of coils and other embolics2
- DMSO compatible microcatheter3
- Radiopaque 0.7 mm platinum/iridium markers allow for rapid and precise positioning4
Progreat 2.4 and 2.0Fr Available in Pre-Shaped Tip Configurations
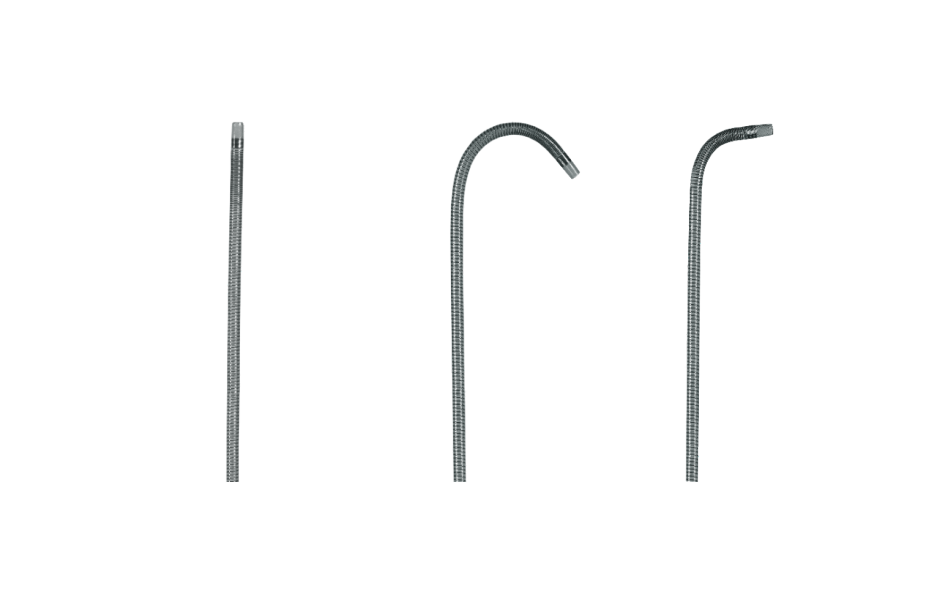
STRAIGHT
J CURVE
70° ANGLE
DESIGNED TO ENHANCE ACCESS TO SMALL PERIPHERAL VESSELS
- TERUMO Glide TechnologyTM hydrophilic coating enhances navigation through tortuous anatomy1,2
- Unique varied tungsten coil pitch construction provides distal flexibility and proximal pushability, enhancing vessel selectivity and catheter placement
PROGREAT® COAXIAL MICROCATHETER SYSTEM
- Available in 2.8Fr and 2.4Fr
- Preloaded with uniquely designed 0.021” GLIDEWIRE® GT for the 2.8Fr system and 0.018" GLIDEWIRE® GT for the 2.4Fr system1
- Enables simultaneous preparation of microcatheter and guidewire, which may save time and increase procedural efficiency1
PROGREAT 2.4 COAXIAL PROVEN TO REACH DISTAL VESSELS WITH EASE⁴
- Provides greater vessel selectivity and demonstrated superiority in traversing tortuous anatomy
- Provides best-in-class kink resistance, demonstrated in both the distal and proximal shaft
PRODUCT CODES
PROGREAT® Microcatheter Product Codes
Includes hemostatic valve and shaping mandrel
| Fr Size | Product Code | Length (cm) | Tip Shape | Max Pressure (psi) |
RO Markers | Hydrophilic Coating Length (cm) |
| 2.4 | MC*PB2411Y | 110 | Straight | 750 | 1 | 50 |
| MC*PB2413Y | 130 | Straight | 750 | 1 | 70 | |
| MC*PB2413ZRA | 130 | 70 Deg. Angle | 750 | 1 | 70 | |
| MC*PB2413ZRC | 130 | J Curve | 750 | 1 | 70 | |
| MC*PV2415Y | 150 | Straight | 750 | 2 | 90 | |
| MC*PV2415ZRA | 150 | 70 Deg. Angle | 750 | 2 | 90 | |
| MC*PV2415ZRC | 150 | J Curve | 750 | 2 | 90 | |
| 2.7 | MC*PC2711Y | 110 | Straight | 750 | - | 50 |
| MC*PC2713Y | 130 | Straight | 750 | - | 70 | |
| 2.8 | MC*PB2811Y | 110 | Straight | 900 | 1 | 50 |
| MC*PB2813Y | 130 | Straight | 900 | 1 | 70 | |
| MC*PV2815Y | 150 | Straight | 900 | 2 | 90 |
PROGREAT® Coaxial Microcatheter System Codes
Includes unique GLIDEWIRE® GT Guidewire, wire stopper, guidewire introducer, hemostatic valve, 2.5 mL syringe, and shaping mandrel
| Fr Size | Product Code |
Length (cm) |
Max Pressure (PSI) |
RO Markers | Hydrophilic Coating Length (cm) |
Glidewire® GT Length (cm) |
Glidewire® GT Size (in) |
| 2.4 Fr | MC*PE24111YB | 110 | 750 | 1 | 50 | 120 | 0.018 |
| 2.4 Fr | MC*PE24131YB | 130 | 750 | 1 | 70 | 140 | 0.018 |
| 2.4 Fr | MC*PE24151YV | 150 | 750 | 2 | 90 | 160 | 0.018 |
| 2.7 Fr | MC*PE27111Y | 110 | 750 | - | 50 | 120 | 0.021 |
| 2.7 Fr | MC*PE27131Y | 130 | 750 | - | 70 | 140 | 0.021 |
| 2.8 Fr | MC*PE28111YB | 110 | 900 | 1 | 50 | 120 | 0.021 |
| 2.8 Fr | MC*PE28131YB | 130 | 900 | 1 | 70 | 140 | 0.021 |
| 2.8 Fr | MC*PE28151YV | 150 | 900 | 2 | 90 | 160 | 0.021 |
PRODUCT SPECIFICATIONS
| Catheter OD | Length (cm) |
Inner Diameter (in/mm) |
Max GW (in) |
Embolic Compatibility |
Dead Space Volume (mL) |
Actual Flow Rate* (mL/sec) @ 750 psi | Actual Flow Rate* (mL/sec) @ 900 psi |
| 2.4/2.9Fr (0.80/0.97mm) |
110 | 0.022"/0.57 | 0.018" | 0.018" Coils/HydroPearl® 600 ± 75 μm |
0.38 | 2.0 | - |
| 2.4/2.9Fr (0.80/0.97mm) |
130 | 0.022"/0.57 | 0.018" | 0.018" Coils/HydroPearl® 600 ± 75 μm |
0.43 | 1.7 | - |
| 2.4/2.9Fr (0.80/0.97mm) |
150 | 0.022"/0.57 | 0.018" | 0.018" Coils/HydroPearl® 600 ± 75 μm |
0.47 | 1.4 | - |
| 2.7/2.9Fr (0.90/0.97mm) |
110 | 0.025"/0.65 | 0.021" | 0.018" Coils/HydroPearl® 800 ± 75 μm |
0.46 | 3.0 | - |
| 2.7/2.9Fr (0.90/0.97mm) |
130 | 0.025"/0.65 | 0.021" | 0.018" Coils/HydroPearl® 800 ± 75 μm |
0.53 | 2.6 | - |
| 2.8/3.0Fr (0.93/1.00mm) |
110 | 0.027"/0.70 | 0.021" | 0.018" Coils/HydroPearl® 800 ± 75 μm |
0.53 | 3.5 | 3.9 |
| 2.8/3.0Fr (0.93/1.00mm) |
130 | 0.027"/0.70 | 0.021" | 0.018" Coils/HydroPearl® 800 ± 75 μm |
0.59 | 3.0 | 3.6 |
| 2.8/3.0Fr (0.93/1.00mm) |
150 | 0.027"/0.70 | 0.021" | 0.018" Coils/HydroPearl® 800 ± 75 μm |
0.66 | 2.7 | 3.2 |
DOCUMENTS
FREQUENTLY ASKED QUESTIONS
What is a microcatheter?
A microcatheter is a very small, flexible catheter designed for navigating through narrow and tortuous blood vessels, typically in neurovascular, peripheral, or coronary interventions. It is usually less than 3 French in diameter and constructed from advanced polymers with braided reinforcement to provide both flexibility and torque control. Microcatheters are used to deliver therapeutic agents—such as embolic materials, contrast media, or medications—or to facilitate the placement of devices like coils or stents in areas that standard catheters cannot reach. Their design allows precise positioning under fluoroscopic guidance while minimizing trauma to delicate vessel walls. In clinical practice, microcatheters are essential for procedures such as embolization of aneurysms, treatment of arteriovenous malformations, and targeted drug delivery.
What are different microcatheter types?
Microcatheters come in several types, each designed for specific clinical applications. Diagnostic microcatheters are primarily used to deliver contrast agents for imaging small vessels. Delivery microcatheters are engineered to transport therapeutic materials such as embolic agents, coils, or stents to targeted sites. Flow-directed microcatheters rely on blood flow for navigation, making them ideal for delicate neurovascular procedures where minimal vessel manipulation is required. Over-the-wire microcatheters use a guidewire for enhanced control and support during complex interventions. Additionally, some microcatheters are dual-lumen, allowing simultaneous infusion and guidewire passage, which is useful in challenging anatomy. Variations also exist in tip shape (straight, angled, or pre-shaped) and coating (hydrophilic for improved trackability), enabling customization based on vessel tortuosity and procedural goals. These design differences ensure precise access and safe delivery in intricate vascular territories.
ASSOCIATED PRODUCTS
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
REFERENCES
- Progreat Microcatheters IFU PG34E002-05 Rev.10 Revised 2023-11
- PROGREAT Catheter 510(k); 2003
- PROGREAT DMSO Compatibility Statement Letter
- Data on file