GLIDEWIRE ADVANTAGE TRACK™ Peripheral Guidewire
Leverage superior performance for your demanding CLI procedures.
Available in 0.018” & 0.014” diameter sizes.

PUSHABILITY
Provides superior pushability to access and cross challenging lesionsa
TRACKABILITY
Provides a superior ability of the guidewire to track and navigate the anatomy of the vasculature systema
DURABILITY
Provides superior shape retention for navigating small, tortuous vesselsa
HYBRID WIRE FOR PAD/CLI
The addition of GLIDEWIRE ADVANTAGE TRACK™ Guidewire to your PAD/CLI toolkit provides you with superior performance1,a for your most challenging cases:
- Superior Pushability: High deformation resistance provides superior pushability to access and cross challenging above-the-knee (ATK) & below-the-knee (BTK) anatomy and lesions
- Superior Trackability: Provides superior ability to track and navigate anatomy of the vasculature
- Best-in-Class Durability: Provides superior shape retention for navigating small, tortuous vessels
- Superior Lubricity: TERUMO Glide Technology™ hydrophilic coatingb provides superior lubricity to support ease of progression through vessels
- Enhanced Visibility & Selectivity: Gold coil tips enhance visibility in conjunction with a 25 cm distal taper designed to minimize vessel trauma and improve selectivity of vessels
a When compared to Abbott Hi-Torque Command 18 LT and Boston Scientific V-18
b Referring to the distal 25 cm of GLIDEWIRE ADVANTAGE TRACK™ wire which features original GLIDEWIRE® construction
TECHNICAL SPECIFICATIONS
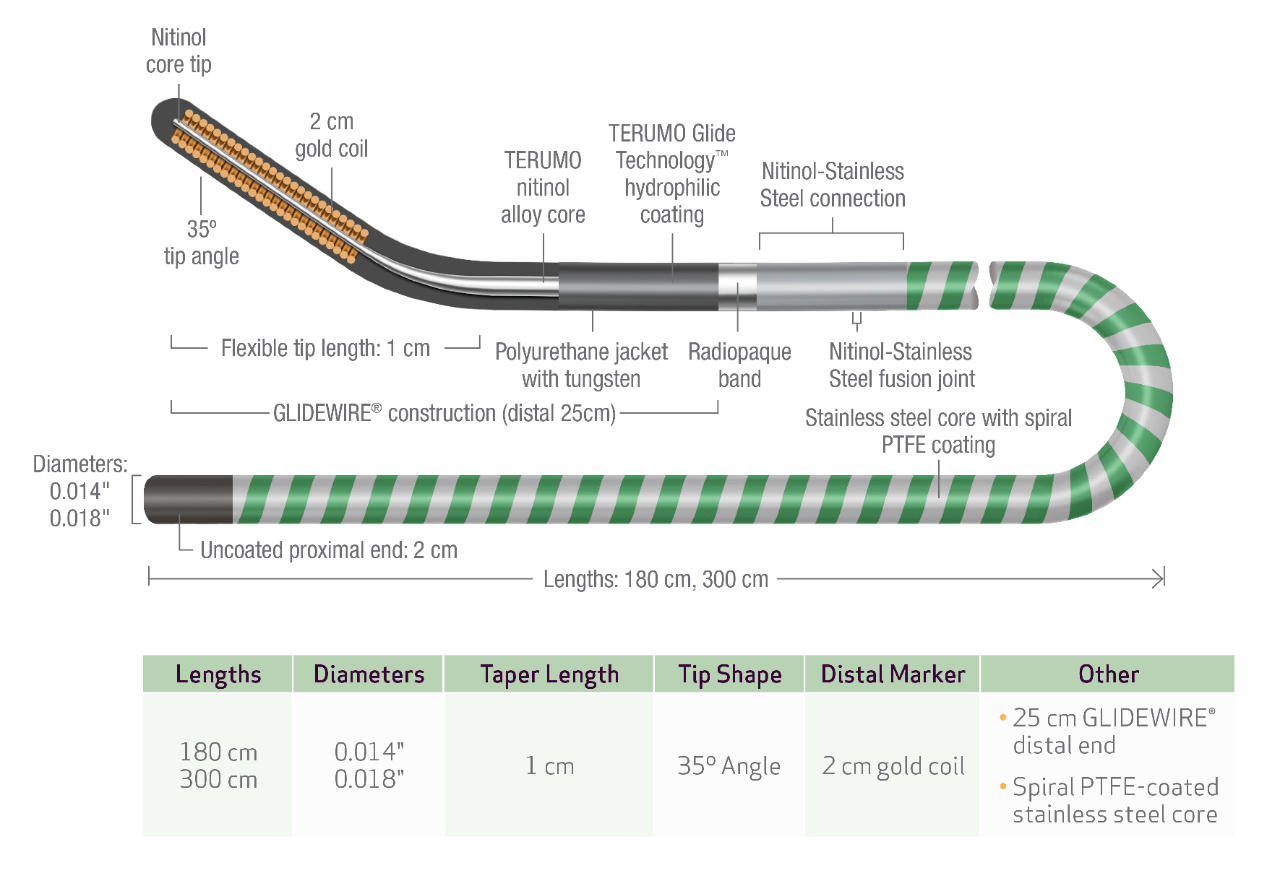
PRODUCT CODES
GLIDEWIRE ADVANTAGE TRACK™ Peripheral Guidewire
| PRODUCT CODE |
DIAMETER | TOTAL LENGTH | DISTAL GLIDEWIRE® LENGTH | FLEXIBLE TIP LENGTH (TAPER) |
TIP SHAPE |
| GAT1418 | 0.014" | 180 cm | 25 cm | 1 cm | 35˚ Angle |
| GAT1430 | 300 cm | ||||
| GAT1818 | 0.018" | 180 cm | |||
| GAT1830 | 300 cm |
DOCUMENTS
ASSOCIATED PRODUCTS
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, potential complications, and instructions for use.
1. Data on file.