PROGREAT ALPHA™
Peripheral Microcatheter
PROGREAT ALPHA™ 2.0 Fr designed to enhance access to small peripheral vessels1,2
CONFIDENTLY DELIVER EMBOLOTHERAPY
- Thin-wall design with large 0.019" lumen¹,²
- Tungsten coil reinforcement for kink resistance³
- DMSO compatible⁴
IMPROVE EFFICIENCY OF EMBOLIZATION PROCEDURES
- Supports the delivery of large embolics—up to 500μm spherical particles, and the AZUR® CX 0.018” Peripheral Coil System²
DESIGNED FOR SMALL VESSEL EMBOLIZATION
- Prostate artery embolization (PAE)
- Pre Y-90 embolization
- Gastrointestinal bleeds
PROGREAT® ALPHA: HIGH-PERFORMANCE PERIPHERAL MICROCATHETER FOR COMPLEX VASCULAR PROCEDURES
Available in unique tip shapes for branch selectivity.
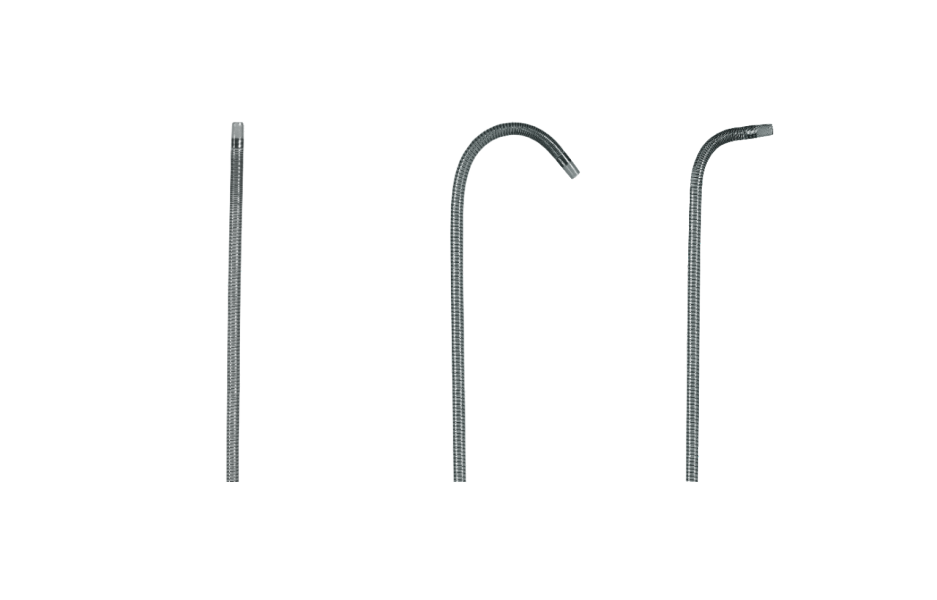
STRAIGHT
J CURVE
70° ANGLE
PRODUCT CODES
PROGREAT ALPHA™ Ordering Information
| Fr Size | Product Code | Length (cm) | Tip Shape | Max Pressure (psi) | RO Markers | Hydrophilic Coating Length (cm) |
| 2.0 | MC*PC2011Y | 110 | Straight | 750 | 1 | 50 |
| MC*PC2013Y | 130 | Straight | 750 | 1 | 70 | |
| MC*PC2013ZRA | 130 | 70 Deg. Angle | 750 | 1 | 70 | |
| MC*PC2013ZRC | 130 | J Curve | 750 | 1 | 70 | |
| MC*PC2015Y | 150 | Straight | 750 | 1 | 90 | |
| MC*PC2015ZRA | 150 | 70 Deg. Angle | 750 | 1 | 90 | |
| MC*PC2015ZRC | 150 | J Curve | 750 | 1 | 90 |
PRODUCT SPECIFICATIONS
PROGREAT ALPHA™ Specifications
| Catheter OD Distal/Proximal (Fr/mm) |
LENGTH (cm) |
INNER DIAMETER (in/mm) |
MAX GUIDEWIRE (in) |
EMBOLIC COMPATIBILITY |
DEAD SPACE VOLUME (mL) |
ACTUAL FLOW RATE* (mL/sec) 750 psi |
| 2.0/2.7 Fr (0.67/0.90mm) |
110 | 0.019/0.49 | 0.016 | AZUR® CX 0.018” Coils/HydroPearl® 400±75 μm |
0.28 | 0.9 |
| 2.0/2.7 Fr (0.67/0.90mm) |
130 | 0.019/0.49 | 0.016 | AZUR® CX 0.018” Coils/HydroPearl® 400±75 μm |
0.32 | 0.8 |
| 2.0/2.7 Fr (0.67/0.90mm) |
150 | 0.019/0.49 | 0.016 | AZUR® CX 0.018” Coils/HydroPearl® 400±75 μm |
0.38 | 0.6 |
DOCUMENTS
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
- Progreat Microcatheters IFU PG34E002-05. Rev. 10 2023-11
- Data on File. Terumo Medical Corporation.
- PROGREAT Catheter 510(k); 2003.
- PROGREAT DMSO Compatibility Statement Letter.