Vascular Access and Closure Devices
Terumo’s portfolio offers a full range of access and closure solutions uniquely engineered to achieve the best possible outcomes.
Terumo Access Devices
Radial Access
GLIDESHEATH SLENDER® Hydrophilic Coated Introducer Sheath
The #1 preferred radial access sheath on the global market*
The GLIDESHEATH SLENDER Sheath has a unique, thin wall design combined with a hydrophilic coating, and featuring a low-profile sheath to reduce arteriotomy size.1,2,3 This enables transradial access without compromise.
Learn more about this hydrophilic-coated introducer sheath
*Data on File
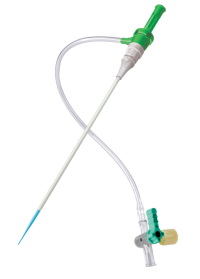
Femoral Access
PINNACLE® PRECISION ACCESS SYSTEM Sheath
The PINNACLE PRECISION Sheath provides smooth, atraumatic access, which has been proven to help reduce complications and enable same-day discharge.4 Total Integrated Fit Technology (TIF) allows for seamless sheath transition which minimizes trauma and supports uncomplicated closing.5
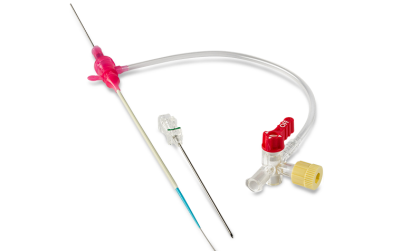
Tibial Pedal Access
GLIDESHEATH SLENDER® Tibial Pedal Kit
Rethink sizing and expand your options for procedural success with Glidesheath Slender’s Tibial Pedal Kit.
Learn more about the proprietary technology in this tibial access kit
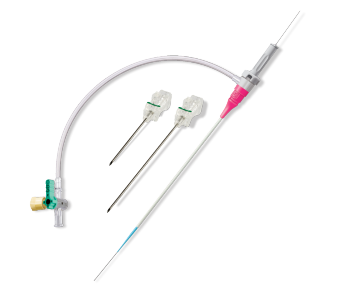
Terumo Closure Devices
Radial Closure
TR BAND® Radial Compression Device
The #1 preferred radial hemostasis device on the global market*
The TR BAND compression device provides precise compression to achieve radial hemostasis at low pressures, minimizing the changes of applying occlusive force6. Innovative design makes the TR BAND extremely easy to use. The unique balloon design allows for better visibility of the green radial artery marker and the redesigned connector piece is the safest non-lueur tip on the market.
Learn more about the TR Band radial approach to hemostasis
*Data on file.
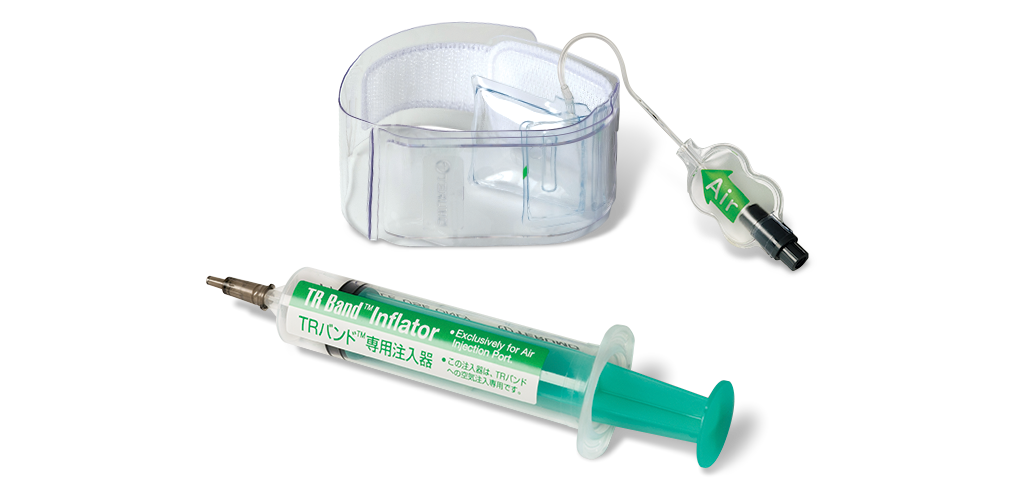
Femoral Closure
ANGIO-SEAL® Vascular Closure Device
The #1 vascular closure device on the global market*
The ANGIO-SEAL device provides active closure for rapid and reliable hemostasis proven to accelerate patient mobility and enable same day discharge. Resorbable components provide immediate closure with uncompromised blood flow 7,8,9
*Data on file.

Important Safety Information:
Possible adverse events for vascular closure devices include, but are not limited to: bleeding or hematoma, AV fistula or pseudoaneurysm, infection, allergic reaction, foreign body reaction, inflammation or edema. This device should only be used by physicians with training qualifying them to perform arterial access and closure for endovascular procedures through the common femoral artery and have participated in a Terumo Medical Corporation Angio-Seal physician instruction program.
Exception (applicable to US and China only): This device should only be used by a licensed physician (or other health care professional authorized by or under the direction of such physician) possessing adequate instruction in the use of the device, e.g., participation in an Angio-Seal physician instruction program or equivalent.
Ref:
1. Saito S, et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Cath Cardio Interv. 1999;46:173-178. 2. Pancholy S. Prevention of radial artery occlusion—patent hemostasis evaluation trial (PROPHET Study): A randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Cath Cardio Interv. 2008;72:335-340. 3. Dahm J, et al. A Randomized Trial of 5 vs. 6 French Transradial Percutaneous Coronary Interventions. Cath Cardio Interv. 2002;57:172–176. 4. Data on file. Terumo Medical Corporation. TIF Engineering Evaluation and Test Production Results #20070045. 5. Data on file. Terumo Medical Corporation. Pinnacle Precision Access System Competitor Evaluation Report #20170018. 6. Ono K. Experience of the TR BAND for the Diagnostic Catheter Procedure. Yuri Union General Hospital in Akita Pref., September 2004. 7. Kapadia SR, Raymond R, Knopf W, et al. Am J Cardiol. 2001;87:789-791. 8. Nash JE, Evans DG. Herz. 1999;24(8):597-606. http://dx.doi.org/10.1007/bf03044483. 9. Angio-Seal® VIP VCD Instructions for Use.
DOCUMENTS
REFERENCES
RX ONLY. This advertisement is directed to physicians only, and not to consumers. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
Indications:
The Angio-Seal Vascular Closure Device is indicated for use in closing and reducing time to hemostasis of the femoral arterial puncture site in patients who have undergone diagnostic angiography procedures or interventional procedures using an 8 French or smaller procedural sheath for the 8 F Angio-Seal device and a 6 French or smaller procedural sheath for the 6 F Angio-Seal device. Angio-Seal is also indicated for use to allow patients who have undergone diagnostic angiography to safely ambulate as soon as possible after sheath removal and device placement, as well as to allow patients who have undergone an interventional procedure to safely ambulate after sheath removal and device placement.
Important Safety Information:
Possible adverse events for vascular closure devices include, but are not limited to: bleeding or hematoma, AV fistula or pseudoaneurysm, infection, allergic reaction, foreign body reaction, inflammation or edema. This device should only be used by physicians with training qualifying them to perform arterial access and closure for endovascular procedures through the common femoral artery and have participated in a Terumo Medical Corporation Angio-Seal physician instruction program.
Exception (applicable to US and China only): This device should only be used by a licensed physician (or other health care professional authorized by or under the direction of such physician) possessing adequate instruction in the use of the device, e.g., participation in an Angio-Seal physician instruction program or equivalent.