ANGIO-SEAL® VIP Vascular Closure Device
The Inside Advantage
Active closure for rapid and reliable hemostasis proven to accelerate patient mobility and enable same-day discharge7-8
How to Deploy Angio-Seal® VIP Vascular Closure Device
STEP-BY-STEP INSTRUCTIONS9
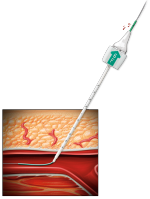
LOCATE THE ARTERY
- Exchange the procedure sheath with the Angio-Seal locator system
- Blood flow through the locator visually confirms proper sheath position in the artery
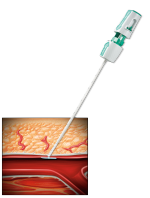
SET THE ANCHOR
- Insert the Angio-Seal VIP device into the sheath until you hear a “click.”
- Gently pull back on the locking cap until you hear another “click.”
- The anchor is now locked in place and device is ready to be deployed.
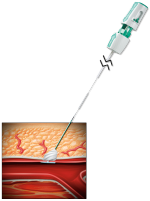
SEAL THE PUNCTURE
- Gently pull back on the Angio-Seal VIP device until the suture has stopped spooling.
- Maintain upward tension on the device and gently advance the compaction tube until resistance is felt.
- Cut the suture and remove the device.
ANGIO-SEAL VIP CLINICAL DEPLOYMENT VIDEOS
Locate the Femoral Artery with Daniel Simon, MD
Clinical Deployment of ANGIO-SEAL® VIP by Daniel Simon, MD
The ANGIO-SEAL® Vascular Closure Device: Set Anchor, Seal Puncture
FREQUENTLY ASKED QUESTIONS
ANGIO-Seal MRI Safety - Is ANGIO-Seal MRI safe?
The implanted components of the device are MRI Safe. The product is not made with natural rubber latex.14
Types of Vascular Closure Devices - What are some common types of vascular closure devices?
Percutaneous Closure Devices: These devices are inserted through a small incision in the skin and are guided to the puncture site to close the artery. They may use sutures, collagen plugs, or other mechanisms for closure.
Collagen Plugs or Sealants: Collagen-based plugs or sealants promote clot formation and provide a physical barrier to reduce bleeding, often used in combination with other closure methods.
Clip-Based Devices: These devices use clips or clamps to secure the arterial puncture site.
Vascular Compression Devices: These devices apply external pressure to the puncture site, aiding in hemostasis. They are commonly used alongside other closure devices.
ANGIO-SEAL VIP PRODUCT CODES
| Part Number | French Size | Guidewire Diameter (in) |
| 610130 | 6Fr | .035 Wire |
| 610131 | 8Fr | .038 Wire |
DOCUMENTS
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
1Kussmaul WG 3rd, Buchbinder M, Whitlow PL, et al. Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. J Am Col Cardiol. 1995;25(7):1685-92. 2.
2Nash JE, Evans DG. The Angio-SealTM hemostatic puncture closure device. Concepts and experimental results. Herz. 1999;24(8):597-606.
3Applegate RJ, Turi Z, Sachdev N, et al. The Angio-Seal Evolution Registry: outcomes of a novel automated Angio-Seal vascular closure device. J Invasive Cardiol. 2010;22(9):420-6
4Aker UT, Kensey KR, Heuser RR, Sandza JG, Kussmaul WG 3rd. Immediate arterial hemostasis after cardiac catheterization: initial experience with a new puncture closure device. Catheter Cardiovasc Diagn. 1994;31(3):228-32.
5Applegate RJ, Rankin KM, Little WC, Kahl FR, Kutcher MA. Restick following initial Angioseal use. Catheter Cardiovasc Interv. 2003;58(2):181-184.
6Tellez A, Cheng Y, Yi GH, et al. In vivo intravascular ultrasound analysis of the absorption rate of the Angio-SealTM vascular closure device in the porcine femoral artery. EuroIntervention. 2010;5(6):731-6.
7Kapadia SR, et al. The 6Fr Angio-Seal arterial closure device: Results from a multimember prospective registry. Am J Cardiol. 2001; 87:789-791.
8Manolis AS, et al. Simplified swift and safe vascular device deployment without a local arteriogram: Single center experience in 2074 consecutive patients. Indian Heart Journal. 2016; 68:529-538.
9Angio-Seal VIP Instructions for Use. ASIN0004. 2018-09-01 4
10StarClose SE IFU EL2101551 (10/2/14)
11MYNX Control Vascular Closure Device IFU 07/21
12MYNXGRIP Vascular Closure Device IFU 04/20
13VASCADE Vascular Closure System IFU 2611 Rev T, 11 AUG 2021
14Perclose ProStyle Suture-Mediated Closure and Repair System IFU EL2127594 (2020-11-03)
15CELT ACD Vascular Closure Device IFU-TS-004 Rev 4 (SCR 230)