HYDROPEARL™ Microspheres
PRODUCT OVERVIEW
DESIGNED FOR CONTROLLED AND TARGETED EMBOLIZATION

UNIQUE FORMULATION
HydroPearl® Microspheres are the first and only Polyethylene Glycol (PEG) microspheres. They are engineered for accurate embolization at the target area.1,2
TIGHT CALIBRATION
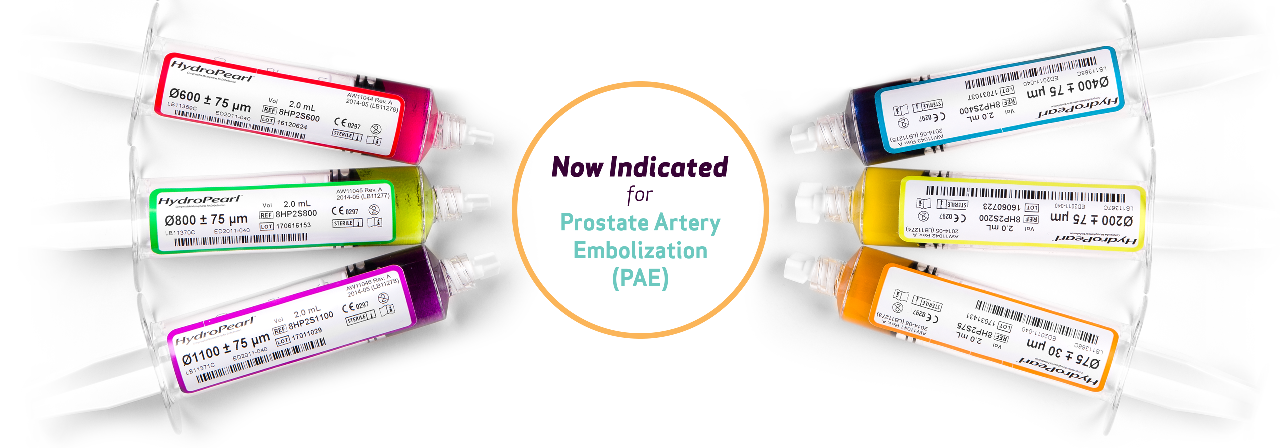
HydroPearl® is available in a range of sizes from 75 μm to 1100 μm with a tighter size distribution*.
This precise calibration may allow more predictable and targeted embolization 1,2**
AVAILABLE IN 6 DISTINCT, COLOR-CODED SIZES
* 90% or higher of HydroPearl sizes are within specification as indicated on the labels.
** Calibration data is 6 lots per each size of randomly selected HydroPearl with 1 lot of competitive products. Sampling done at minimum 150 microspheres per syringe.
HydroPearl Video – Robustness
HydroPearl® microspheres are intended for the embolization of: 3,4
- Hypervascular tumors
- Symptomatic uterine fibroids
- Arteriovenous malformations (AVMs)
- Prostatic Arteries (PAE) for symptomatic benign prostatic hyperplasia (BPH)
IN-SERVICE VIDEO
PRODUCT CODES
HYDROPEARL® Microspheres
| HYDROPEARL SIZES (μm) | PRODUCT CODES | VOLUME OF MICROSPHERES (ml) |
MICROSPHERE COLOR |
| 75 ± 30 μm | HP2S0075 | 2 | Orange |
| 200 ± 75 μm | HP2S0200 | 2 | Yellow |
| 400 ± 75 μm | HP2S0400 | 2 | Blue |
| 600 ± 75 μm | HP2S0600 | 2 | Red |
| 800 ± 75 μm | HP2S0800 | 2 | Green |
| 1100 ± 75 μm | HP2S1100 | 2 | Purple |
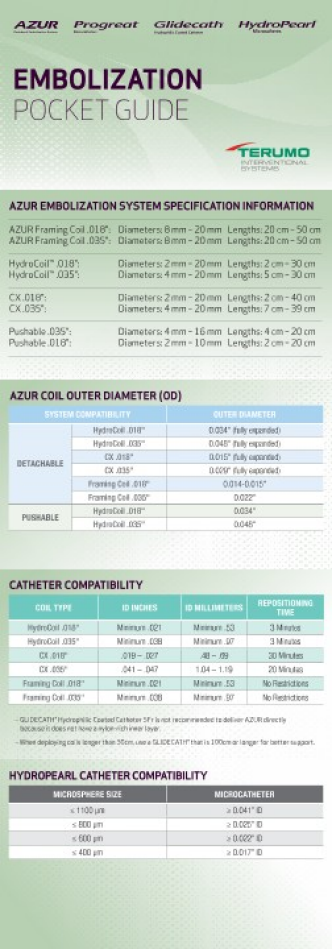
TERUMO CATHETER COMPATIBILITY1
| HYDROPEARL SIZES (μm) | PROGREAT® 2.0F | PROGREAT® 2.4F | PROGREAT® 2.7F | PROGREAT® 2.8F | GLIDECATH® 4.0F |
| 75 ± 30 μm | Compatible | Compatible | Compatible | Compatible | Compatible |
| 200 ± 75 μm | Compatible | Compatible | Compatible | Compatible | Compatible |
| 400 ± 75 μm | Compatible | Compatible | Compatible | Compatible | Compatible |
| 600 ± 75 μm | Non-Compatible | Compatible | Compatible | Compatible | Compatible |
| 800 ± 75 μm | Non-Compatible | Non-Compatible | Compatible | Compatible | Compatible |
| 1100 ± 75 μm | Non-Compatible | Non-Compatible | Non-Compatible | Non-Compatible | Compatible |
DOCUMENTS
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
- Data on file.
- Karolin J. Paprottka et al. In vitro study of physical properties of various embolization particles regarding morphology before, during and after catheter passage. Clinical Hemorheology and Microcirculation. 1386-0291/16.
- Per Instructions For Use PD111835 Rev. B Revised 2020-02
- HydroPearl 510(k) Letter. 22 Jan. 2020