AZUR™ CX Peripheral Coil System
PRODUCT OVERVIEW
OPTIMIZE OUTCOMES IN MORE PROCEDURES
2mm and 3mm sizes are available for small vessel embolization


SOFT, FLEXIBLE AND PRECISE FOR CONTROLLED DELIVERY
- Mechanical occlusion — less reliance on thrombus formation1
- Soft feel with up to 30 minutes repositioning time*

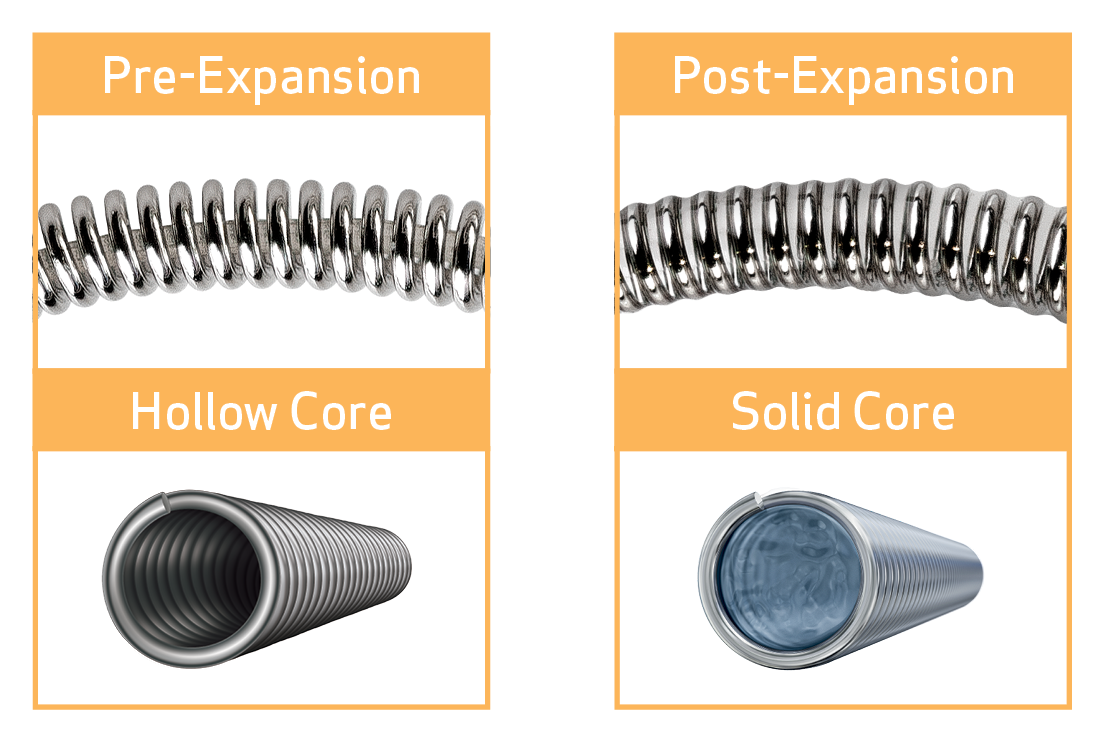
DESIGNED TO FORM A SOLID CORE
- Enables expansion between the gaps with Hydrogel that will not be absorbed by the body1,2
EASE OF DEPLOYMENT
- Use the AZUR® Detachment System for precise positioning and placement
- Minimize catheter manipulation with unique coil design3

EXPAND YOUR OPTIONS
The introduction of 2 and 3 mm sizes enables AZUR® CX to support more procedures, including:
SMALL VESSEL EMBOLIZATION
- Gastrointestinal bleeds
- Pre-Y-90 embolization
- Prostate artery embolization
- Other pelvic vasculature embolizations
LARGE VESSEL EMBOLIZATION
- Internal Iliac Procedures
- Pelvic congestion syndrome
- Varicocele embolization
- Peripheral Aneurysms
- Arteriovenous malformations
Treat more patients with the potential to use fewer coils3

TAKE CONTROL
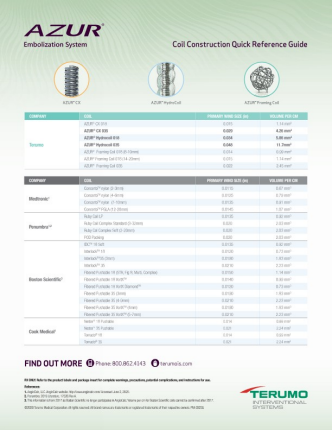
Choose from a comprehensive line of peripheral coils from TERUMO**
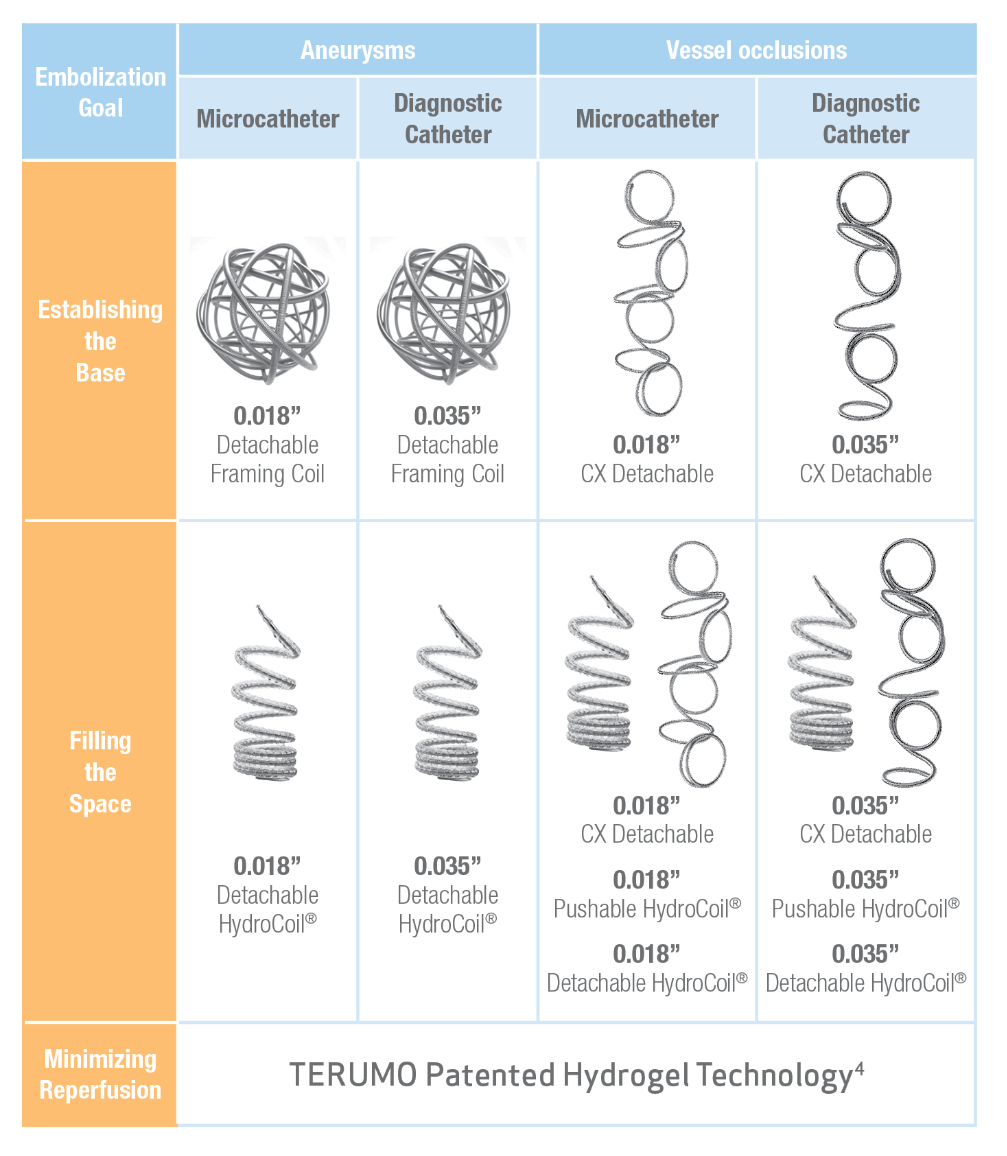
**For every vessel occlusion or aneurysm where a microcatheter or standard diagnostic catheter is required or preferred
IN-SERVICE VIDEO
PRODUCT CODES
AZUR® CX Detachable 0.018” System / Pack of 1 - Product SKUs
| PRODUCT CODE | LOOP DIAMETER (mm) |
LENGTH† (cm) |
| 45-780202 | 2 | 2 |
| 45-780204 | 2 | 4 |
| 45-780304 | 3 | 4 |
| 45-780308 | 3 | 8 |
| 45-780413 | 4 | 13 |
| 45-780516 | 5 | 16 |
| 45-780620 | 6 | 20 |
| 45-780724 | 7 | 24 |
| 45-780828 | 8 | 28 |
| 45-780928 | 9 | 28 |
| 45-781032 | 10 | 32 |
| 45-781238 | 12 | 38 |
| 45-781434 | 14 | 34 |
| 45-781639 | 16 | 39 |
| 45-781836 | 18 | 36 |
| 45-782040 | 20 | 40 |
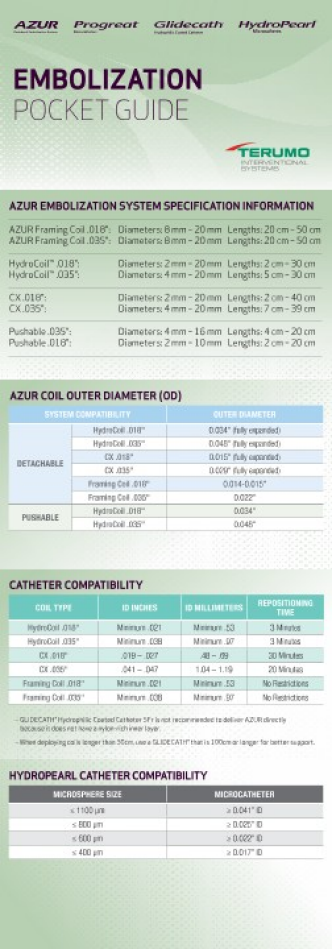
CATHETER ID REQUIREMENTS
| COIL TYPE | INCHES (min-max) |
MILLIMETERS (min-max) |
REPOSITIONING TIME |
| AZUR® CX 0.018" | .019 - .027 | .48 - .69 | 30 minutes |
| AZUR® CX 0.035" | .041 - .047 | 1.04 - 1.19 | 20 minutes |
DOCUMENTS
AZUR® CX Detachable 0.035” System / Pack of 1 - Product SKUs
| PRODUCT CODE | LOOP DIAMETER (mm) |
LENGTH† (cm) |
| 45-750407 | 4 | 7 |
| 45-750511 | 5 | 11 |
| 45-750609 | 6 | 9 |
| 45-750617 | 6 | 17 |
| 45-750812 | 8 | 12 |
| 45-750824 | 8 | 24 |
| 45-751019 | 10 | 19 |
| 45-751324 | 13 | 24 |
| 45-751632 | 16 | 32 |
| 45-752039 | 20 | 39 |
Detachable Controller for use with Detachable Systems / Pack of 5
| PRODUCT CODE | PRODUCT DESCRIPTION |
| 45-4001 | AZUR® Detachment Controller |
REFERENCES
RX ONLY. Refer to the product labels and package insert for complete warnings, precautions, potential complications, and instructions for use.
- Pelage JP. Angiographic and Pathologic Comparison of HydroCoils vs. Fibered Coils Mechanisms of Occlusion and Mid-Term Recanalization in an Animal Model. GEST. 2012 (animal study)
- Plenk H, Killer M, Richling B. Pathophysiologic considerations on HydroCoil- and platinum coil-occluded retrieved human cerebral aneurysms. Presented at ASITN MicroVention Symposium. 2005 (in-vivo study)
- Data on File. Terumo Corporation. IMS Data.
*Instructions For Use PD110323 Rev. C Revised 2014-04.